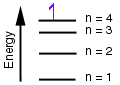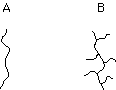|
Tools - Chemistry ConcepTests: Sample Set #1
Arthur Ellis
Department of Chemistry
1101 University Ave. Madison WI 53706
University of Wisconsin-Madison
email: ellis@chem.wisc.edu
608-262-0421
Introduction:
Readers may be familiar with Harvard physicist Eric Mazur's ConcepTests, which have been shown to enhance substantially the comprehension of introductory physics concepts. Briefly, conceptual questions are posed in the lecture room along with a few possible answers. Students vote on the possible answers, then try to persuade their neighbors in the lecture room that they are correct, and finally vote again. This form of peer instruction is often an effective pedagogical method, and it also provides the instructor with on-line feedback as to how well the class is following the lecture.
The ConcepTests include in parenthesis the concepts being addressed and the chapter they correspond to from the book, "Teaching General Chemistry, A Materials Science Companion," Arthur B. Ellis, et al., American Chemical Society, 1993.
Click here to see a second set of sample chemistry ConcepTests or visit the chemistry ConcepTest website at http://www.chem.wisc.edu/~concept/.
Show the answers.
Chemistry ConcepTests: Sample Set #1
States of Matter, Solids, Liquids, Gases, Colloids, and Solutions
1. (Ideal gas law) Consider direct and inverse relationships among ideal gas law variables.
Demonstration: A hard-boiled egg is placed over the opening of an Erlenmeyer flask. What will happen to each gas law variable when the flask is placed in a tub of liquid nitrogen?
number of gas moles: goes up, goes down, stays constant
temperature: goes up, goes down, stays constant
volume of trapped gas: goes up, goes down, stays constant
pressure of trapped gas: goes up, goes down, stays constant
What will happen to the egg?
nothing, it will pop out of the flask, it will be sucked into the flask
Electronic Structure and Periodicity
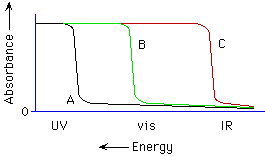
2. (Spectroscopy; Ch. 7 "Companion") Knowing diamond is colorless, which curve best represents the absorption spectrum of diamond (see below)?
3. (Energy diagrams) According to the energy diagram below for the Bohr model of the hydrogen atom, if an electron is in the 4th energy level, how many electronic transitions are expected as the electron falls to lower energy levels?
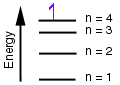
4. (Electromagnetic radiation, spectroscopy)
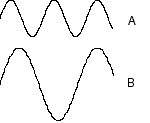
Which wave below has a higher frequency?
A, B, both have the same frequency
Which wave below has a larger wavelength?
A, B, both have the same wavelength
Which wave below has a higher energy
A, B, both have the same energy
Thermochemistry
5. (Enthalpy, equilibrium) For the reaction:
N2(g) + 3H2(g)  2NH3(g) + reaction energy 2NH3(g) + reaction energy
Which have collectively stronger bonds?
those in the reactants, those in the products
Kinetics & Mechanism
6. For the reaction:
2NO2(g)  2NO(g) + O2(g) 2NO(g) + O2(g)
Each of the following curves corresponds to one of the species in the reaction shown above. Which curve represents the time dependence of the concentration of O2?
Bonding and Structure
7. (Polymers) Some polymerization reaction conditions lead largely to linear chains of polyethylene, A, while other conditions cause considerable branching, B. Which structure corresponds to high density polyethylene (HDPE)?
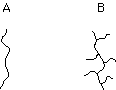
Chemical Reactions, Acid-Base, Redox, Precipitation
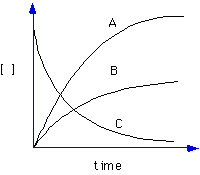
8. (Limiting reagents, precipitation, solubility)
Demonstration: A solution of Ba(NO3)2 is added to a solution of Na2SO4 to make a precipitate, barium sulfate, BaSO4.
The amount of precipitate collected from the fixed amount of Na2SO4 solution as the Ba(NO3)2 is added indefinitely will look like which graph below?
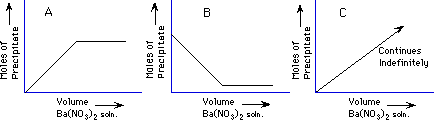
The break point of the graph will occur when the ratio of moles of Ba+2 to moles of SO4-2 is
Other
9. (Thermal conductivity; Ch. 7 "Companion") If students have a seat/desk with metal and wooden parts that are not in direct contact with their bodies, ask about the relative temperature: Which is colder the wooden or metal parts?
wood, metal, both are at the same temperature
Demonstration: Measure the temperature of each part with a digital thermometer. (Tie in with thermal equilibrium and the calculated value of ambient thermal energy as RT.)
If the temperature of the room goes from 20 oC to 40 oC, the ambient thermal energy
doubles, is halved, increases by less than 10%
At-Seat Demonstration 7.5 "Companion": Touch the wood and metal part of the desk. Which
material conducts heat better and thus has a higher thermal conductivity?
wood, metal, the two materials are the same

|